The basic unit of all matter is the atom. Atoms are made of three basic parts called protons electrons and neutrons.
 Atoms Atoms Are The Building Blocks For The Whole Universe Ppt Video Online Download
Atoms Atoms Are The Building Blocks For The Whole Universe Ppt Video Online Download
What chemical laws can be explained by Daltons theory.

Atoms the building blocks of matter. The Building Blocks of Matter From Philosophical Idea to Scientific Theory Particle theory of matter Democritus 400 BC Called natures basic particle an atom based on the Greek word meaning indivisible Aristotle Didnt believe in atoms Thought all matter was continuous His opinion was accepted for nearly 2000 years There was no experimental evidence to back up the views of. A neutral atom has the same number of electrons as it does protons. Distinguish between atomic number and mass number.
Atoms cannot be subdivided created or destroyed. The Building Blocks of Matter Atoms. THE BUILDING BLOCKS OF MATTER 71 Materials can covered by a sock sealed with tape one or more objects that fit in the container metric ruler balance Wear safety goggles and an apron.
In other words an atom of each element is different from an atom of any other element. This chapter introduces the fundamental building blocks of matter and some of the important classification schemes scientists use to communicate about matter. Atoms of different elements combine in small whole number ratios to.
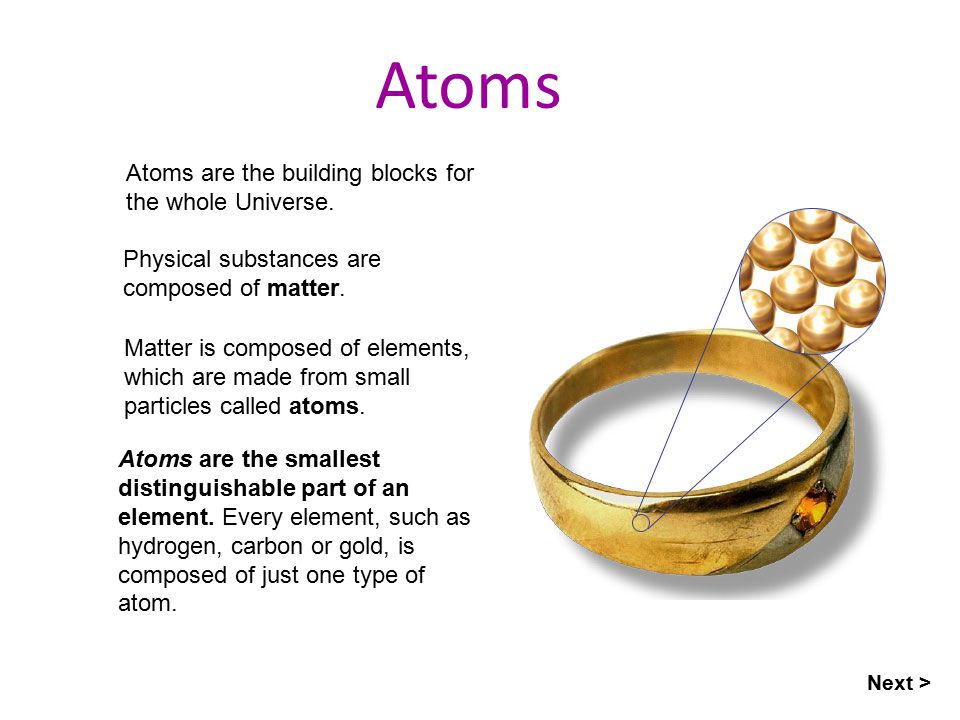
Elements such as helium depicted here are made up of atoms. Atom is often confused with molecule and the distinction between element and compound is also one that learners. 60gK 17gL xgK 85gLx30 PTS.
Atoms combine in whole number ratios so their proportion by mass will always be the same. Atoms of different elements differ in some fundamental way. Atoms of different elements differ in.
From Philosophical Idea to Scientific Theory 1. The mass of the products is different than the mass of the reactants. Identify the key distinction between isotopes of the same element.
Discuss the relationships between matter mass elements compounds atoms and subatomic particles. Atoms molecules mixtures and compounds in urduatoms in urduatoms and molecules class 9atomsatoms and moleculesatoms class 12 physics wallahatoms shoes. Atoms cannot be subdivided created or destroyed.
All matter is composed of extremely small particles called atoms. Several changes have been made to. All matter is composed of extremely small particles called atoms.
O is always made of 2 hydrogen and 1 oxygen. Atoms consist of a positively charged nucleus made up of protons and neutrons that is surrounded by a negatively charged electron cloud. All matter is composed of tiny indivisible particles called atoms 2.
LODP results from the fact that a given chemical compound is always composed of the same combination of atoms. Atoms of a given element are identical in size mass and other properties. The Building Blocks of Matter Section.
Constant composition implies constant properties. You will learn what makes atoms th. The Building Blocks of Matter.
The Development of a New Atomic Model 1. Atoms of a given element are identical in size mass and other properties. Explain how electrons occupy electron shells and their contribution to an.
They are all made of these same basic parts they differ in how many of them they have. List the five main points of Daltons atomic theory. So the mass ratio.
Explain how an atom can exist in this state. However even the atom can be broken into smaller pieces called quarks. Matter changes when reacted or changed.
The mass of the reactants is the same as the mass of the products. The Stucture of the Atoms 1. The mass of all reactants are changed during a physical or chemical change.
Atoms of different elements combine in simple-whole number ratios to form chemical compounds In chemical reactions atoms are combined separated or rearranged. Atoms of different elements differ in size mass and other properties. Atoms are the smallest particle of pure matter that can exist on its own and is stable there are smaller bits but they go around causing trouble as they arent stable.
Atoms cannot be subdivided created or destroyed. Chapter 3 test - Atoms. One of the main challenges of this introduction and this is true at all levels is that learners are easily confused by the terminology.
All matter is composed of extremely small particles called atoms. Is always 162 or 81. In this video you will learn about the three basic building blocks of all types of matter--atoms molecules and crystals.
THE BUILDING BLOCKS OF MATTER 19. C 4 Arrangement of Electrons in Atoms Section. An atom that has lost or gained any electrons.
The Building Blocks of Matter Answer Section MULTIPLE CHOICE 1. The positive and negative charges combine to form a net neutral charge. Atoms of an element are identical in size mass and other properties.
By the end of this section you will be able to. Atoms of a given element are identical. The atom is the smallest unit of matter that cant be divided using any chemical means and the building block that has unique properties.
Atoms are made up of protons and neutrons located.
Halaman
OnTheIssues
Cari Blog Ini
Label
- 1939
- 1950s
- 1970
- 2000
- 2012
- 2014
- 2015
- 2016
- 2017
- 25th
- 4runner
- 550i
- abeja
- aberdeen
- abortar
- abortion
- about
- academy
- accident
- account
- aciclovir
- actors
- adam
- address
- adrenal
- adultos
- africa
- african
- after
- again
- aids
- airline
- airlines
- airplanes
- airport
- alabama
- album
- albuquerque
- aldean
- algodon
- alice
- alloa
- alternatives
- amazon
- america
- american
- americana
- americano
- amos
- amsterdam
- amyotrophic
- andy
- anesthesia
- angeles
- angels
- animal
- animals
- años
- another
- answers
- anti
- aparatos
- apes
- aplicacion
- apps
- arabia
- arabian
- araña
- arenas
- argentina
- arms
- army
- arnold
- arris
- arsenal
- arte
- artists
- artritis
- asap
- aseguranza
- ashley
- asian
- atlanta
- atlantic
- atomic
- atoms
- atraer
- attack
- audi
- auditions
- aumentar
- austin
- australian
- awake
- away
- axon
- azucar
- babies
- baby
- back
- backpacks
- bags
- bahamas
- bajar
- bajo
- ball
- balloon
- balls
- band
- bandits
- bangs
- bank
- banned
- bans
- barbed
- barbie
- barcelona
- barrilito
- bars
- bases
- basketball
- baskets
- bass
- bateria
- batman
- bayou
- beach
- bear
- bears
- beat
- beauty
- bebe
- beckham
- becky
- become
- been
- before
- beijing
- being
- bells
- belt
- beneficios
- benefit
- benz
- beowulf
- berners
- bernie
- best
- bethesda
- beverly
- bible
- biggest
- bike
- bikila
- bird
- birth
- bitcoin
- black
- blackstar
- blake
- blancos
- blanquear
- bleaching
- block
- blocks
- bloks
- blossom
- blue
- bluefin
- board
- body
- books
- boots
- booty
- boss
- bottle
- bottles
- bottoms
- bowie
- boys
- braid
- braids
- brain
- brand
- brands
- brasileño
- brazilian
- break
- breakfast
- breathing
- breed
- bridges
- bristol
- broadway
- bros
- brother
- brown
- bruno
- buen
- bueno
- build
- building
- bulb
- bull
- buscar
- business
- cabello
- cabrio
- caillou
- calendario
- california
- call
- calling
- cambogia
- cameras
- canal
- canas
- cancer
- candidatos
- canvas
- caps
- caption
- cara
- card
- cards
- cargar
- caribbean
- carrera
- carro
- carvings
- cascos
- case
- cases
- catch
- catholics
- cause
- caves
- celebrities
- celular
- celulas
- cena
- center
- centre
- centro
- centrum
- cerveza
- cessna
- challenge
- champions
- chance
- chanel
- changes
- chapo
- charger
- chastity
- chat
- chateau
- chavo
- cheap
- cheating
- cheats
- chekhov
- cherry
- chest
- chica
- child
- children
- chile
- china
- chinchillas
- chinese
- chocolate
- choo
- christian
- chrysler
- church
- cigars
- cinema
- citizenship
- city
- clasico
- classic
- clear
- clinton
- clip
- clocks
- clockwork
- clothes
- clothing
- club
- clubs
- coast
- coat
- cocinar
- colageno
- colonial
- coloring
- comedia
- comezon
- como
- company
- comprar
- compras
- computer
- concert
- concerts
- conch
- concrete
- condoms
- connection
- conservative
- considered
- consulado
- consulate
- contra
- control
- convenience
- converse
- cooker
- coral
- corona
- corsets
- cosmetics
- cost
- costco
- costume
- costumes
- cough
- count
- counter
- county
- couples
- course
- court
- covenant
- cover
- cowboys
- craft
- cream
- creature
- cremas
- crew
- cristiano
- crosstrek
- cruises
- cual
- cuando
- cuanto
- cuantos
- cuba
- cuello
- cuisine
- culver
- cups
- curcumin
- cure
- customs
- daily
- dalai
- dallas
- damage
- dame
- dancer
- dark
- darker
- darth
- data
- date
- dates
- dating
- daughter
- david
- dead
- death
- dedo
- deleted
- delivery
- deportados
- desde
- designer
- desk
- desmayar
- despues
- detector
- dice
- diego
- dies
- different
- diprospan
- direct
- directors
- disorder
- doctor
- doctors
- doctrinas
- documentary
- does
- dogs
- dolar
- dolls
- dolor
- dolphin
- domestic
- dominican
- dominicana
- donald
- donation
- door
- dormir
- downloads
- dread
- dream
- dress
- drinking
- drown
- dubai
- dublin
- duplo
- dura
- dusseldorf
- east
- easy
- ebay
- edgar
- edge
- edible
- edith
- edition
- educacion
- effective
- egyptian
- ejercicio
- ejercicios
- election
- electric
- electricity
- elephant
- elephants
- ellas
- ellie
- elmer
- embarazadas
- embarazo
- embarazos
- embassy
- emblem
- emirates
- empire
- empty
- encias
- engagement
- engine
- england
- episode
- episodes
- escape
- esclavas
- escribe
- español
- espia
- espiritual
- espn
- essay
- estado
- estados
- estomago
- estudio
- etta
- euro
- european
- euthanasia
- everton
- evidence
- exchange
- exhibit
- exotic
- expensive
- experience
- extension
- extensiones
- extinction
- face
- facing
- fall
- family
- famosos
- famous
- fashion
- fast
- february
- female
- ferguson
- ferns
- feyenoord
- fighters
- figurehead
- final
- find
- fingers
- finish
- fire
- first
- fish
- five
- flag
- flamingo
- flat
- flema
- flies
- flight
- flights
- florida
- food
- foods
- football
- force
- ford
- forecast
- formula
- fort
- forum
- fotos
- foxes
- foyles
- fraiche
- francisco
- free
- french
- friday
- from
- fuga
- full
- funciona
- funeral
- funk
- furniture
- futbol
- future
- galaxy
- gallery
- game
- games
- gana
- ganglion
- garage
- gemelos
- gender
- gentelmans
- gentlemens
- geodesic
- german
- gestacion
- gets
- getting
- gift
- giraffes
- girdles
- girls
- giselle
- giving
- gland
- glasgow
- glass
- global
- gnomes
- gold
- golds
- golf
- good
- gorilla
- government
- granada
- grand
- gratis
- great
- grey
- gripe
- grocery
- groton
- groups
- grow
- guardar
- guatemala
- guess
- guinea
- guinness
- guns
- guys
- hablas
- hacemos
- hacer
- hacerse
- hack
- hair
- haircut
- half
- hall
- halloween
- hallway
- hambre
- hamburg
- hamburger
- hamilton
- hammer
- harry
- hatred
- have
- having
- head
- healthy
- heart
- heat
- heathers
- heel
- heels
- helicopter
- hello
- helmet
- helmets
- hermine
- hero
- higado
- high
- hillary
- hills
- hips
- holiday
- hollywood
- holy
- hombre
- hombres
- hombros
- home
- homeopathic
- honduras
- hookah
- horror
- horse
- hotels
- hours
- house
- houston
- hoverboards
- hovertrax
- howard
- huddle
- hulu
- human
- humans
- hurt
- hysterectomy
- ideas
- ikea
- illusions
- image
- imagenes
- images
- immigration
- immunocal
- india
- indian
- indiana
- indies
- indigenous
- indocumentados
- inflamada
- Information
- ingles
- injustice
- inmigracion
- interchangeable
- international
- interview
- into
- inverness
- inyectable
- iphone
- ireland
- iron
- isis
- islam
- island
- israeli
- italy
- items
- itunes
- jack
- jacket
- jackson
- jade
- jaguar
- jail
- james
- jeans
- jennifer
- jerry
- jersey
- jessie
- jesus
- joan
- jobs
- joel
- johannesburg
- john
- johnsons
- jong
- jordan
- jose
- juega
- juegos
- july
- jumping
- kahlo
- kampf
- kate
- kennedy
- kids
- kill
- killing
- kimball
- king
- kings
- kitchen
- kittens
- kitty
- knee
- know
- kobe
- kodak
- korean
- kruger
- labor
- laborales
- ladies
- lady
- laid
- lamp
- lana
- land
- laptops
- late
- lateral
- latin
- latinas
- latitude
- lawn
- laws
- lead
- league
- learn
- leather
- leche
- lego
- lens
- letters
- levantar
- leve
- level
- lewis
- licencia
- life
- light
- lighthouse
- like
- limpieza
- line
- live
- living
- llamar
- llena
- lloyd
- local
- lockers
- loco
- london
- long
- look
- looks
- lopez
- loss
- louisiana
- love
- lowering
- lucky
- lululemon
- luna
- lyric
- lyrics
- mackey
- madrid
- make
- makes
- makeup
- male
- malnatis
- manchester
- manos
- many
- mapa
- maps
- marathons
- marcar
- march
- maria
- marihuana
- mark
- maroon
- marotta
- mary
- masks
- masses
- masturbating
- matter
- mavic
- mayor
- mayores
- mean
- meaning
- meat
- medica
- medicamentos
- medicina
- medicinas
- medico
- medieval
- medio
- meet
- mega
- mein
- mejor
- memorial
- memphis
- menlo
- mens
- menu
- mercedes
- mercer
- meses
- metal
- mexican
- mexicanos
- mexico
- mezquinos
- miami
- miata
- michael
- mickelson
- mighty
- miley
- millie
- minecraft
- ming
- mini
- minks
- miss
- mobile
- montauk
- months
- montreal
- morgan
- mosquito
- mosquitos
- most
- mother
- motor
- motors
- move
- movie
- much
- muerte
- mugshots
- mujer
- mummy
- mundo
- muriendose
- music
- musica
- musical
- nacidos
- nacional
- nail
- name
- national
- natural
- naturales
- navy
- near
- nest
- netflix
- newark
- news
- newspapers
- nigerian
- night
- nights
- nike
- nikki
- niño
- ninos
- nissan
- nitrous
- niveles
- nombres
- norethindrone
- northampton
- norwegian
- nose
- note
- november
- nuevo
- number
- numbers
- numero
- ocean
- october
- ocultar
- ohio
- oido
- oils
- oklahoma
- oldest
- oneplus
- online
- open
- opera
- orange
- orleans
- osteen
- ostrich
- ouji
- outfits
- oxide
- padre
- page
- painting
- paintings
- palabras
- panama
- panel
- pant
- panties
- pants
- para
- paradise
- paris
- parte
- parto
- parts
- party
- paso
- pastillas
- pasts
- patriots
- patties
- pearls
- peliculas
- pelo
- pennsylvania
- people
- perfect
- perfumes
- periodico
- periods
- perkin
- peru
- peso
- peta
- phase
- phoenix
- phone
- photo
- photography
- pianos
- pics
- picture
- pictures
- piel
- pies
- pillow
- pills
- ping
- pink
- pitbull
- pizza
- place
- plan
- plane
- planet
- plans
- plastic
- plata
- play
- playas
- plus
- point
- poirot
- pokemon
- poland
- polar
- poldark
- political
- pomada
- poner
- pong
- poppies
- popstar
- posiciones
- positive
- potato
- potter
- powder
- power
- precio
- precios
- pregnancy
- pregnant
- preguntas
- preparing
- prescriptions
- presidencia
- president
- price
- prices
- priests
- prince
- princess
- privadas
- prix
- producers
- produces
- pros
- prostata
- prostate
- protection
- proteina
- prueba
- psoriasis
- puerto
- pull
- pumpkins
- pure
- purring
- python
- queen
- quinceañera
- quiz
- quotes
- race
- radiation
- rain
- rams
- rappers
- rate
- reading
- real
- reality
- reasons
- recall
- record
- redneck
- regal
- reindeer
- relaciones
- related
- relationship
- relationships
- release
- religious
- remedio
- remedios
- remedy
- remote
- rent
- repelente
- republic
- rescue
- residencies
- responders
- rest
- restaurants
- results
- retirement
- reumatoidea
- reveal
- review
- reviews
- revolution
- rezos
- rica
- richmond
- rico
- riff
- ring
- river
- rivers
- roach
- road
- roblox
- robot
- rock
- rocky
- rodilla
- rome
- ronaldo
- rooney
- ropas
- rothko
- royal
- runner
- russia
- saber
- sale
- salon
- salt
- samsung
- sanctuary
- sangrado
- santa
- sarandon
- satanica
- satelital
- satelite
- saudi
- save
- saxophone
- scar
- schedule
- schizoaffective
- schoenberg
- sclerosis
- scooters
- scotland
- screen
- script
- seafood
- seal
- sealed
- season
- second
- seeking
- sell
- semanas
- sentence
- september
- service
- sets
- seven
- shades
- sheet
- shells
- shelter
- shelters
- shelton
- ship
- shipping
- shirt
- shirts
- shoes
- shoots
- short
- shorthaired
- show
- side
- sign
- significado
- simons
- simply
- sing
- singapore
- sintomas
- sioux
- sirve
- site
- size
- sketcher
- skirts
- slide
- slogans
- smart
- smash
- sneeze
- snow
- soccer
- soldier
- solomon
- somali
- someone
- song
- songs
- sonic
- sons
- sorry
- sort
- soundtrack
- sourdough
- south
- spade
- spanish
- speak
- spears
- specialist
- spell
- spice
- sporting
- sports
- spotify
- springs
- spurs
- squeeze
- stabbing
- stagg
- stained
- standing
- star
- state
- station
- statues
- sterling
- stick
- stickers
- stimulation
- stock
- store
- stores
- stories
- story
- straight
- stratosphere
- stream
- strong
- structure
- studio
- stun
- subaru
- subatomic
- suburbs
- success
- sudamerica
- suerte
- suit
- suits
- sunedison
- sunset
- super
- superman
- surgery
- sutton
- swearing
- swift
- sydney
- symbol
- symbolism
- syndrome
- syrup
- tabla
- tail
- taking
- talkies
- tall
- tank
- target
- tattoo
- tattoos
- taylor
- tech
- teddy
- telefono
- telefonos
- temperanceville
- temperature
- temple
- temporary
- tener
- tennis
- tent
- tentacles
- tercera
- terminal
- territory
- test
- tests
- texas
- that
- there
- they
- thomas
- three
- thrones
- through
- thyroid
- tickets
- tight
- time
- times
- tiredness
- tissue
- titanic
- today
- toilet
- tomar
- toms
- tongue
- torre
- toshiba
- touched
- touchscreen
- tour
- toyota
- toys
- trabajo
- traductor
- trailer
- train
- training
- transcranial
- transforms
- transmitted
- trap
- tratamiento
- travel
- treat
- tree
- trench
- tribe
- tricks
- tropical
- truck
- trump
- trumpet
- trust
- tulsa
- tumblr
- tuna
- type
- uber
- uggs
- ugly
- understand
- underwear
- unidos
- union
- united
- universal
- universe
- used
- vader
- vamos
- vegas
- velocity
- venezolano
- venezuela
- vernon
- vestidos
- vibram
- vibrators
- vida
- video
- videos
- vina
- vineyard
- vintage
- vinyl
- violence
- virgin
- virtual
- viruses
- visa
- vitamin
- vitaminas
- vivo
- voodoo
- vote
- walk
- walking
- walmart
- want
- warming
- warriors
- wars
- wasp
- watch
- watchers
- water
- wayfair
- wear
- weather
- weave
- weaver
- weaves
- wedding
- week
- welsh
- west
- western
- whales
- what
- when
- where
- which
- while
- whipped
- whirlpool
- whisky
- white
- whole
- wiccan
- wigs
- will
- window
- wire
- with
- witness
- wizard
- wolves
- woman
- women
- womens
- word
- words
- working
- worksheet
- world
- worlds
- worth
- wounds
- xbox
- xylitol
- yarmouth
- year
- years
- yertle
- yoga
- york
- young
- your
- yquem
- zealand
- zombie
- zoomie
- zurich
- คน
- ไทย
- ใน
